Although there are no hard and fast rules, the general rule is if the difference in electronegativities is less than about 0.4, the bond is considered nonpolar; I'll tell you the polar or nonpolar list below. If the difference is greater than 0.4,.
SOLVED 0.3 In water, MgCl2 dissociates into Mg2+ and Cl. Based on
A polar molecule with two or more polar bonds must have an asymmetric.
Is mgcl2 a polar covalent bond a covalent bond or an ionic bond?
Mgcl2 is polar because its molecular geometry is bent, it’s not symmetrical thus leading mgcl2 being polar since the dipole forces are pulling downwards. The bond polarity between two atoms can be estimated if. Hence, we have a polar molecule. If you want to quickly find the word you want to search, use ctrl + f, then type the word you want to search.
Mgcl2 is a polar molecule, like water. So, the gist is that polar substances readily dissolve polar substances. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. List molecules polar and non polar

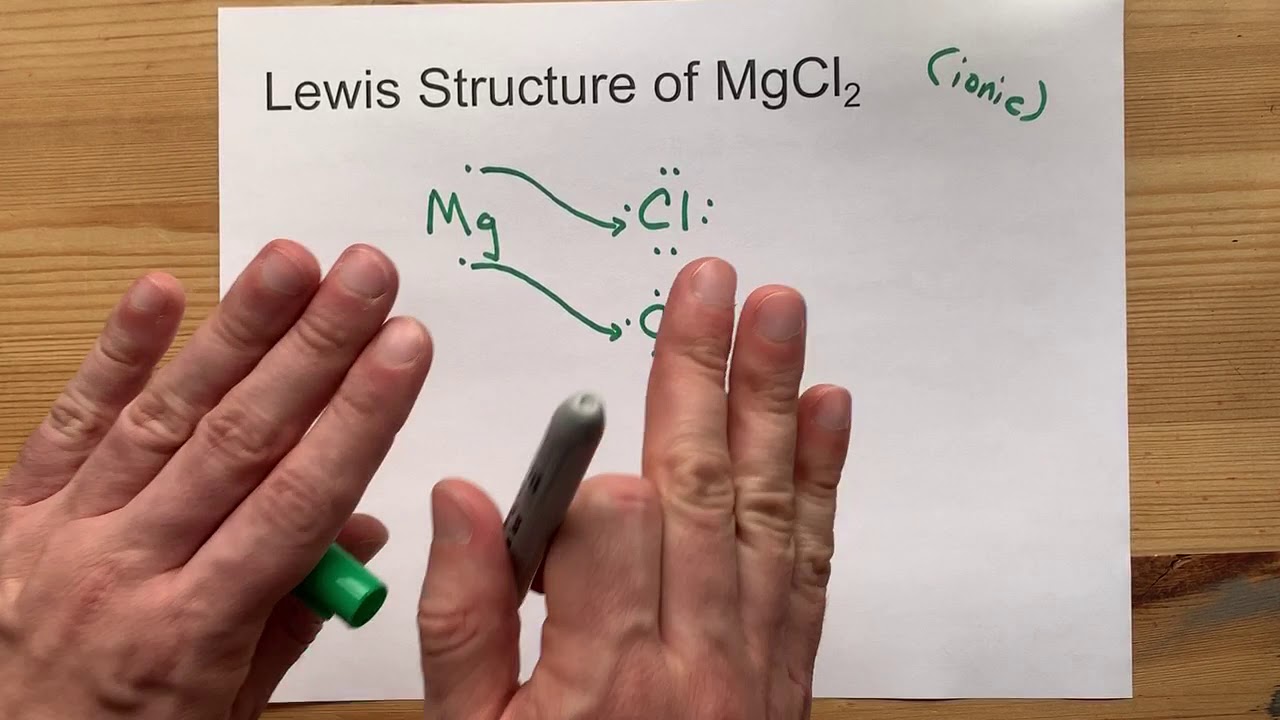
Mgcl2 forms an ionic bond, where magnesium (mg) transfers electrons to chlorine (cl) resulting in the.
Is mgcl2 polar or nonpolar?