The more complex an element, the more neutrons, protons and. It also has one electron in. Find out what is the simplest atomic structure, how to discover it, and what are the different kinds of atoms.
Basic Atomic Structure Science, Atoms And Elements ShowMe
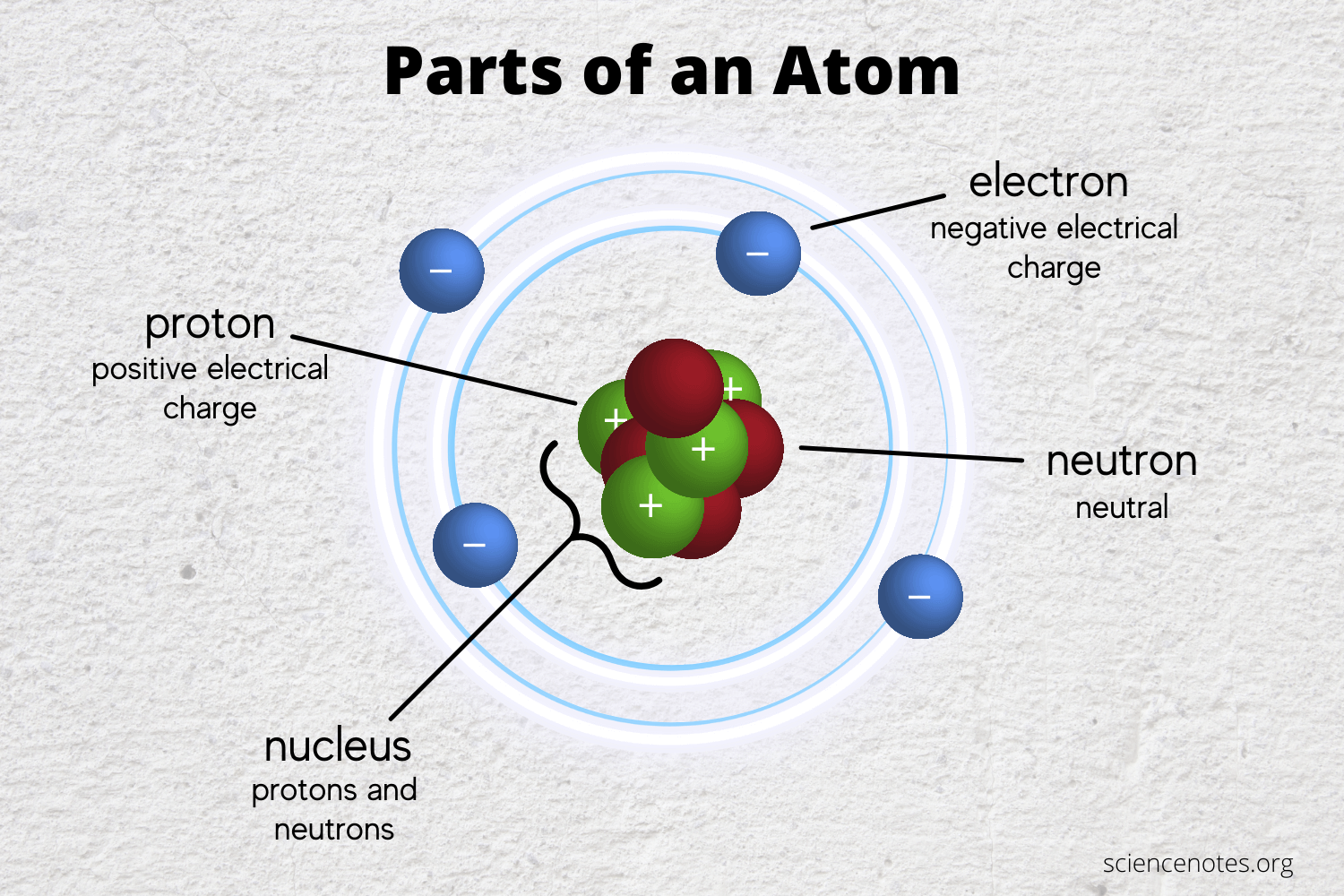
Moving around the nucleus in a cloud of possible positions are electrons.
The structure of the atom.
The simplest atom is that of hydrogen: Hydrogen is considered to have the simplest atomic structure because it has an atomic number of 1, meaning it has only one proton in its nucleus. Hydrogen (not carbon) forms more compounds than any other element! A hydrogen atom consists of one proton and one electron.
1 electron and one proton. The correct option is d. Atomic structure is a fundamental concept within physical chemistry that deals with the study of the structure of an atom. They are known as elements.
Quarks, charged leptons, neutrinos, the.
Learn the structure of atoms from the atomic models by. Basically, the atomic structure refers to the structure of. Atoms have protons and neutrons in the center, making the nucleus, while the electrons orbit the nucleus. In every stable, neutrally charged atom there is the exact same number of protons as electrons.
The simplest atomic structure is hydrogen. Atomic structure or structure of atom consists of a nucleus having protons and neutrons, electrons revolve around it. The structure of a hydrogen atom can be thought of as like the earth and its moon. It consists of only one proton and one electron, making it the most basic element.

Learn the terms and concepts of atomic structure with this set of flashcards.
When we break matter down to its most fundamental components, we find that there are many different types of indivisible particles: There are many different atoms, each having its name, size, mass, and number of subatomic particles. At the center of the atom is a tiny “nucleus”, a core made up of protons and neutrons.


